Abstract

Purpose: Myelofibrosis (MF) is a clonal stem-cell derived BCR-ABL negative myeloproliferative neoplasm characterized by atypical megakaryocytic proliferation leading to bone marrow reticulin and collagen fibrosis. About 90% of patients with MF harbor somatic driver mutations that constitutively activate the JAK-STAT pathway. There have been other high molecular risk genes identified (ASXL1, EZH2, IDH1, IDH2, SRSF2) that predict inferior survival and have been added to prognostic scoring systems (Cazzola et al, Blood 2014).
RAS pathway activating mutations are common in myeloid malignancies. Mutations in NRAS or KRAS themselves have been reported in ~5% of MF cases, and are associated with more proliferative disease behavior, decreased overall survival, and decreased chances of response to JAK inhibitor therapy (Coltro et al, Blood Adv 2020; Santos et al, Leukemia 2020). Downstream mutations in other RAS pathway genes such as NF1, BRAF, CBL, or PTPN11 are relatively common in MF, although their impact on disease behavior or response to therapy is not yet well characterized. Here, we aim to characterize clinical features of RAS pathway activated MF, describe response to therapies including JAK inhibitors, and determine impact on patient outcomes.
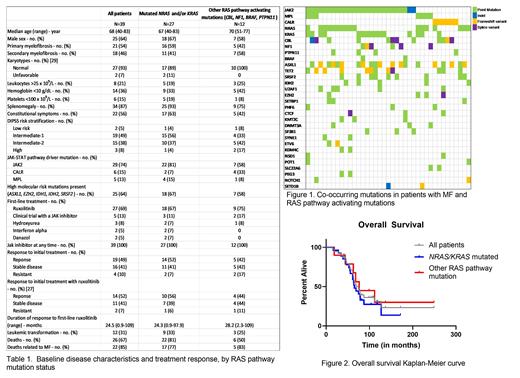
Methods: We retrospectively analyzed the medical records of 118 consecutive adult patients with myelofibrosis who were seen at our tertiary referral center between July 2011 and July 2021 and who had next generation sequencing (NGS) performed - either MiOncoseq (Roychowdhury et al, Sci Transl Med 2011) or Tempus myeloid NGS. We grouped patients by those with mutated NRAS and/or KRAS (n=27) and those without mutated NRAS or KRAS but with another RAS pathway activating mutation (CBL, NF1, BRAF, PTPN11; n=12).
Results: Of our cohort with MF, 39 (33%) harbored a RAS pathway activating mutation: NRAS, n=17; KRAS, n=15; CBL, n=12; NF1, n=6; BRAF, n=2; PTPN11, n=4. Fifteen (38%) patients had at least 2 different RAS pathway mutations. All of these patients had a JAK2 (n=29), CALR (n=6) or MPL (n=5) mutation in addition to their RAS pathway mutation.
Ruxolitinib was the most common first-line MF directed therapy in 27 patients (69%); other less common first-line treatments included clinical trials containing a JAK inhibitor (5), hydroxyurea (3), interferon alpha (2), or danazol (2). With first-line therapy, overall response rate by IWG-MRT criteria was 49% (n=19), 16 (41%) had stable disease, and 4 (10%) had resistant disease. There was no statistical difference in type of response to first-line ruxolitinib between the NRAS/KRAS mutated group and the other RAS pathway mutated group (p=0.8). Twenty-seven patients went on to receive second-line therapy - 14 patients on a clinical trial with a JAK inhibitor. Twelve patients (31%) received at least 3 unique lines of treatment.
After median follow-up of 69 months, 13 (33%) were alive and 26 (67%) had died, with 22 of these deaths related to their myelofibrosis diagnosis. Median overall survival was 74.7 months, 65.3 months in the NRAS/KRAS mutated group, and 76.2 months in the other RAS pathway mutated group, with no difference in overall survival between the two groups (p=0.72). Twenty patients (74%) from the NRAS/KRAS mutated group had a cause of death related to MF compared to 6 patients (50%) in the other group. Overall, 12 patients (31%) had leukemic transformation, 9 (33%) in the NRAS/KRAS mutated group and 3 (25%) in the other RAS pathway mutated group (p=0.72).
Conclusion: Most patients with RAS pathway mutations presented with intermediate risk clinical characteristics (based on the DIPSS risk status), splenomegaly, and constitutional symptoms. Among those treated first-line with JAK inhibitors, about 50% of patients responded to therapy, similar to prior studies looking at JAK inhibitors in MF, though duration of response was short in both of the RAS activated subgroups (24 months and 28 months). In comparing patients with direct NRAS/KRAS mutations to those with other RAS pathway mutations, there was no difference between risk status at diagnosis, treatment responses overall and to ruxolitinib specifically, or overall survival. While our sample size was limited, our data suggests that patients with direct NRAS/KRAS mutations had similar clinical features and outcomes compared to those with other RAS pathway mutations. Further studies with larger cohorts are warranted to help predict response to JAK inhibitors.
Talpaz: Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy; Imago: Consultancy; Constellation: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: Grant/research support .
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal